The UW Pulmonary Division is a leader in airway immunopathology, particularly translational asthma research. The major focus of the program is on understanding the immunological basis of a key component of asthma called airway hyperresponsiveness (AHR). This form of airway dysfunction is the critical source of symptoms in most individuals with asthma and can be utilized to understand the potential therapeutic responses to asthma therapy. This area is integrated with the other major paradigm in asthma focusing on the inflammatory endotype but is uniquely positioned to better understand the relation to physiological airway dysfunction and airway epithelial dysfunction. Asthma research is conducted through a highly collaborative local group of investigators that includes our Division and well-developed collaborative programs with the UW Allergy Section, Seattle Children’s Research Institute (SCRI) and Benaroya Research Institute (BRI). This program includes one of the Asthma & Allergic Diseases Cooperative Research Centers led by Dr. Teal Hallstrand that is based out of the UW Pulmonary Division and funded through the National Institute of Allergy and Infectious Diseases. In addition, there is a substantial body of NIH funding, focusing on asthma immunology, lipid mediators, epithelial biology, and the function of innate cells, particularly mast cells and eosinophils.
Translational Research
Researchers from the Pulmonary Division, including Drs. Teal Hallstrand and Ryan Murphy, have conducted multiple cohort studies that examine the basis for AHR in asthma and uncovered the major mechanisms involved in a feature of AHR called exercise-induced bronchoconstriction. This work has led to the discovery of several novel targets for the development of future therapy and directly addresses the role of airway dysfunction in asthma. Multiple informative repositories of airway samples and primary cell culture are available in existing repositories in addition to those that are in progress. These studies involve the careful characterization of clinical phenotype and endotype, linking airway dysfunction to immunopathology, using flow cytometry, lipid mediator analysis, ex vivo cellular analysis often using live primary airway cells, and more.
Genomics and Bioinformatics
At the origin of this program, the use of state-of-the-art bioinformatics has been at the core of the mission of biological discovery, leading to the identification of a typical mast cell gene signature associated with airway dysfunction and that these cells are in the airway epithelial compartment (intraepithelial mast cells). This program continues to expand through the leadership of Dr. Sina Gharib, the Director of Bioinformatics at the Center for Lung Biology and Dr. Matthew Altman, who directs the asthma bioinformatics team at the Center for Systems Biology at BRI. This program is highly successful because of the access to informative cohorts locally and through networks, availability of primary airway and epithelial samples and ex vivo models, creating a rich environment for discovery using multiple techniques including bulk and single cell RNA sequencing. Further examination of epigenetic changes and genetic variants related to epithelial dysfunction have led to multiple key discoveries in asthma.
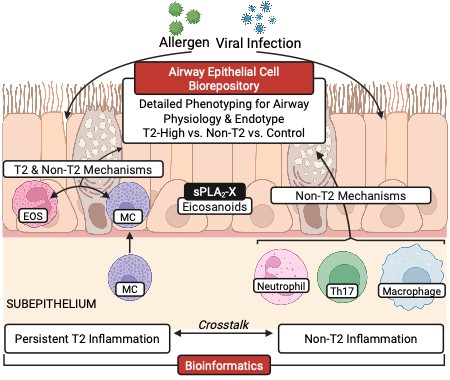
Epithelial Biology
Over the last 15 years, the UW has been the leader in understanding the role of the airway epithelium in asthma. The original work in this area came from the labs of Dr. Hallstrand in collaboration with several other program including the lab of Dr. Jason Debley at SCRI. These programs have created a link between the manifestations of asthma and epithelial dysfunction and have established some of the best developed airway epithelial cell (AEC) repositories using airway brushing-derived epithelial cells from well-characterized children and adults with and without asthma. This work of discovery led to an annual conference of local collaborators, including the Seattle based investigators at the UW, SCRI, BRI and with regional members from Oregon Health Sciences University (OHSU) and the University of British Columbia (UBC). This meeting has been highly supportive environment leading to many collaborations and multiple successful trainees.
Pre-clinical Research
Pre-clinical models of asthma have been developed through collaborations with Drs. Bill Altemeier, Michael Gelb (Biochemistry) and Marion Pepper (Immunology) that are based on the findings from human translational studies. These studies address the role of secreted phospholipase A2s (sPLA2), mast cells, and IL-1 family members including IL-33. The program contains novel models of asthma that are designed to better replicate the features of human disease. The program has developed or obtained many informative murine models including specific transgenic mice and targeted deletions. A notable example from the program is the finding that deletion of the sPLA2 group 10 gene (Pla2g10), encoding a protein that is markedly elevated in human asthma, eliminates the allergen-induced rise in leukotrienes that are critical mediators of inflammation in asthma.
Quantitative Imaging
Quantitative analysis is facilitated through the Histology and Imaging Core at South Lake Union. Airway endobronchial biopsy samples from well characterized individuals have been obtained in a manner that facilitates the use of three-dimensional quantitative imaging in the form of stereology. This cohort is the largest well defined cohort examining airway dysfunction that is currently available. These studies have revealed the precise location of mast cells and eosinophils in the airway wall. Ongoing work is being conducted that examines the role of epithelial remodeling in asthma and airway epithelial neuronal innervation.






